- Construction
- Industry
- References
- Our Services
- Worth knowing
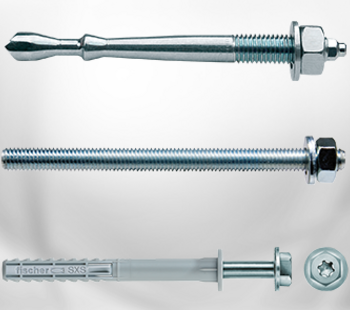
In 2002, Wilhelm Modersohn became aware of the SAF2304 (1.4362) material through Avesta in Sweden. In 2003 Modersohn introduced the first Lean Duplex steel for system products in the German market for fastening systems.
- Downloads
- Company

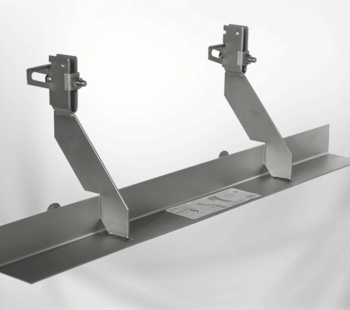
As an independent representative of fastening systems from the company Lutz from Wertheim, Wilhelm Modersohn sen. had recognised early on that there was a great demand in the market for fastenings in the area of masonry facades. In March 1970, he and two employees founded the company, which today has over 140 employees.
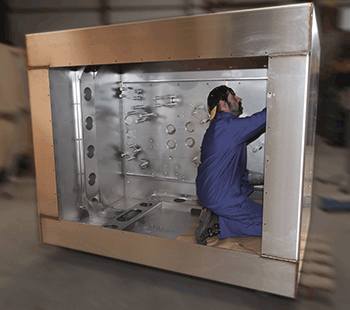
Electropolishing
In electropolishing, material is removed on an electrochemical basis, with the help of a current source based on direct current. In an electrolyte that is specially adapted to the metal to be processed, anodic removal of the surface takes place.
The result is a reduced surface roughness as well as a lowered micro-roughness, which produces a metallurgically pure, shiny surface without imperfections.
Performance data
- Useful size of basin 1: 3,000 x 700 x 750 mm (length x width x filling height)
- Useful size of basin 2: 800 x 450 x 500 mm (length x width x filling height)
Procedure
- The workpieces to be polished must be freed from oils, greases, oxides and other contaminants that would hinder even electropolishing
- They are then mounted on titanium, copper or bronze frames. Polishing is done by immersing them in the polishing bath with the electrolytes and applying current. This removes material from the surface. The process is a reversal of electroplating, where metal ions from a solution are attached to the material. The metal removal is proportional to the applied current, the effectiveness of the electrolytes and the treatment time.
- An increased current is applied to burrs or other protruding irregularities, so that these are preferentially removed.
- The dwell time in the bath depends on the material, type and surface of the workpieces used.
- Afterwards, the fabric removed from the polishing bath is rinsed to remove any polishing electrolytes still adhering to it.